BE AWARE OF COUNTERFEIT BOTOX® COSMETIC
Beware of counterfeit and adulterated BOTOX® Cosmetic. Unfortunately, there are medical offices and injectors who are using counterfeit BOTOX® Cosmetic. It is not uncommon for offices to get faxes, solicitation emails and phone calls from people trying to represent themselves as certified sellers of different aesthetic supplies at reduced rates.
On August 4, 2015, two Canadians were sentenced for distributing counterfeit, misbranded and adulterated BOTOX® Cosmetic into the United States. This case was investigated by the FDA’s Office of Criminal Investigations. One defendant received a sentence of 24 months of imprisonment, and the other received a sentence of 3 months. They operated a wholesale drug distribution business selling non-FDA approved BOTOX® Cosmetic sourced from Turkey to U.S. doctors. Besides being illegal to use in the United States, the BOTOX® Cosmetic was not stored or shipped refrigerated, had counterfeit exterior packaging and manufacturing lot numbers on the cartons did not match the lot numbers on the drug vials inside the cartons.
On that same day, Philippe Schaison, President of Allergan Medical Aesthetics, issued a letter to Arizona Aesthetic Health Care Partners. He stated that illegally imported or counterfeit BOTOX® Cosmetic posed a significant risk to patient safety because the quality and safety of the drug is unknown and cannot be guaranteed. Because of this safety issue, the Arizona Legislature passed Arizona House Bill 2322 that became effective July 3, 2015, making it a felony offense to manufacture, sell or distribute misbranded drugs in Arizona. A drug is misbranded if it is not approved by the FDA or is obtained outside the licensed supply chain regulated by the FDA, the Board of Pharmacy, or the Department of Health Services.
What does this mean for patients? First of all, be cautious if the cost of your BOTOX® Cosmetic per unit is suspiciously lower than the average cost in your area. Could a decreased cost per unit be the result of the provider purchasing their supplies from an illegal source at a lower cost to them? Remember most offices charge by the unit and your treatment cost should be the cost per unit times the number of units used. Secondly, check the reputation of the office or injector you are using. If there have been complaints about poor results, could it be that a knock-off product is being used rather than authentic BOTOX® Cosmetic or just bad technique by the injector? An office or injector that uses illegally obtained BOTOX® Cosmetic risks being criminally prosecuted by the FDA Office of Criminal Investigations. Thirdly, ask to see the BOTOX® Cosmetic vial. Only authentic BOTOX® Cosmetic vials have a sophisticated hologram image that states “Allergan”. The BOTOX® Cosmetic carton will also indicate if the product is packaged for use in the United States by a U.S. License Number (1145) located on the box side panel as well as on the product vial.
Patients who suspect that they are or were being treated with illegal BOTOX® Cosmetic can anonymously report their concerns to www.authenticbotoxcosmetic.com or to the FDA Office of Criminal Investigations (OCI) by calling 1-800-551-3989, or visiting the OCI Website.
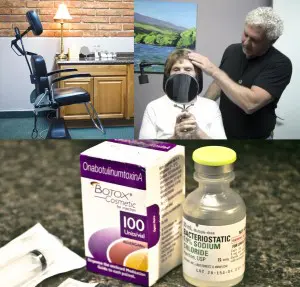
In my practice here at Willo MediSpa, I only purchase and use aesthetic products that come directly from their respective manufacturers. The BOTOX® Cosmetic I use is purchased from and supplied by Allergan. The vials are shipped overnight via FedEx Express packaged in a Styrofoam container with dry ice to keep the temperature controlled. In the office, the product is kept in a freezer until it is reconstituted with bacteriostatic saline for patient use.
Also, I regularly show new patients how BOTOX® Cosmetic is packaged, the vial it comes in and what it looks like before being reconstituted to make it injectable. I find that this information gives patients some extra understanding and reassurement about their treatment.
Please contact me if you have any questions concerning BOTOX® Cosmetic by email at [email protected], by phone at 602-296-4477 or stop by the office at 201 E. Monterey Way, Phoenix, AZ 85012.

Dr. Nello Rossi – Willo MediSpa
Phoenix, AZ 85012
Phone: (602) 296-4477
Email: [email protected]
Website: https://willomedispa.com
Office Hours
Sunday – Monday: Closed



 SkinMedica® Store
SkinMedica® Store